ASTRO will feature a Contouring Case of the Day. Each day of the meeting, join us in reviewing a new case. Each case will include a case description and four possible scenarios with the question "How would you contour for this patient?"
Following each day's Contouring Case of the Day, an answer and rationale will be given by the presenters of that case. Would you have contoured the same way?
eContouring Case of the Day
Daily Cases
Saturday, September 27, 2025
Contouring for Gynecologic Cancer
Review Saturday's case on cervical cancer and review the four possible contouring scenarios to let us know how you would contour.
Faculty:
- Jenna Kahn, MD - Kaiser NW Permanente
- Kevin Albuquerque, MD - Simmons Comprehensive Cancer Center, UTSW Radiation Oncology
Sunday, September 28, 2025
Contouring for Genitourinary Cancer
Review Sunday's case on prostate cancer and review the three possible contouring scenarios to let us know how you would contour.
Faculty:
- Hong Zhang, MD, PhD - URMC Wilmot Cancer Institute
- Neil Taunk, MD, MS - University of Pennsylvania
Monday, September 29, 2025
Contouring for Central Nervous System Cancer
Review Monday's case on gliomas and review the three possible contouring scenarios to let us know how you would contour.
Faculty:
- Michelle Kim, MD - University of Michigan
- Helen Shih, MD, MS, FASTRO, MPH, AM - Massachusetts General Hospital
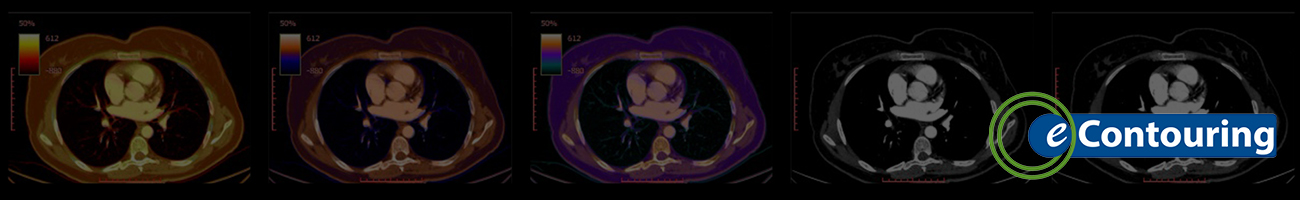
Tuesday, October 1, 2025
Contouring for Thoracic Cancer
Review Tuesday's case on thoracic cancer and review the three possible contouring scenarios to let us know how you would contour.
Faculty:
- Pooja Karukonda, MD - Duke Cancer Institute
- Daniel Gomez, MD, MBA, FASTRO - Memorial Sloan Kettering Cancer Center
Acknowledgements
Thank you to the eContouring Case of the Day planners for putting together this year's program.
- Salma Jabbour, MD, FASTRO - Rutgers University
- Manisha Palta, MD - Duke Cancer Institute
